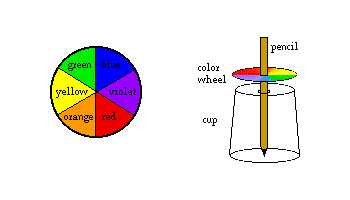Why is the sky blue?
On a clear sunny day, the sky above us looks bright blue. In the evening, the sunset puts on a brilliant show of reds, pinks and oranges. Why is the sky blue? What makes the sunset red?
To answer these questions, we must learn about light, and the Earth's atmosphere.
THE ATMOSPHERE
The atmosphere is the mixture of gas molecules and other materials surrounding the earth. It is made mostly of the gases nitrogen (78%), and oxygen (21%). Argon gas and water (in the form of vapor, droplets and ice crystals) are the next most common things. There are also small amounts of other gases, plus many small solid particles, like dust, soot and ashes, pollen, and salt from the oceans.
The composition of the atmosphere varies, depending on your location, the weather, and many other things. There may be more water in the air after a rainstorm, or near the ocean. Volcanoes can put large amounts of dust particles high into the atmosphere. Pollution can add different gases or dust and soot.
The atmosphere is densest (thickest) at the bottom, near the Earth. It gradually thins out as you go higher and higher up. There is no sharp break between the atmosphere and space.
LIGHT WAVES
Light is a kind of energy that radiates, or travels, in waves. Many different kinds of energy travel in waves. For example, sound is a wave of vibrating air. Light is a wave of vibrating electric and magnetic fields. It is one small part of a larger range of vibrating electromagnetic fields. This range is called the electromagnetic spectrum.
Electromagnetic waves travel through space at 299,792 km/sec (186,282 miles/sec). This is called the speed of light.
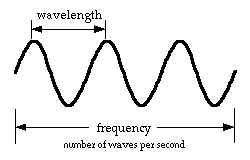
The energy of the radiation depends on its wavelength and frequency. Wavelength is the distance between the tops (crests) of the waves. Frequency is the number of waves that pass by each second. The longer the wavelength of the light, the lower the frequency, and the less energy it contains.
COLORS OF LIGHT
Visible light is the part of the electromagnetic spectrum that our eyes can see. Light from the sun or a light bulb may look white, but it is actually a combination of many colors. We can see the different colors of the spectrum by splitting the light with a prism. The spectrum is also visible when you see a rainbow in the sky.
The colors blend continuously into one another. At one end of the spectrum are the reds and oranges. These gradually shade into yellow, green, blue, indigo and violet. The colors have different wavelengths, frequencies, and energies. Violet has the shortest wavelength in the visible spectrum. That means it has the highest frequency and energy. Red has the longest wavelength, and lowest frequency and energy.
LIGHT IN THE AIR
Light travels through space in a straight line as long as nothing disturbs it. As light moves through the atmosphere, it continues to go straight until it bumps into a bit of dust or a gas molecule. Then what happens to the light depends on its wave length and the size of the thing it hits.
Dust particles and water droplets are much larger than the wavelength of visible light. When light hits these large particles, it gets reflected, or bounced off, in different directions. The different colors of light are all reflected by the particle in the same way. The reflected light appears white because it still contains all of the same colors.
Gas molecules are smaller than the wavelength of visible light. If light bumps into them, it acts differently. When light hits a gas molecule, some of it may get absorbed. After awhile, the molecule radiates (releases, or gives off) the light in a different direction. The color that is radiated is the same color that was absorbed. The different colors of light are affected differently. All of the colors can be absorbed. But the higher frequencies (blues) are absorbed more often than the lower frequencies (reds). This process is called Rayleigh scattering. (It is named after Lord John Rayleigh, an English physicist, who first described it in the 1870's.)
WHY IS THE SKY BLUE?
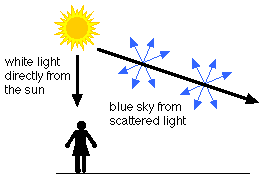
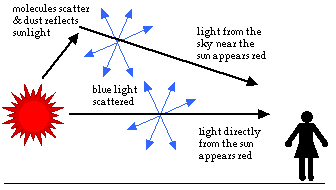
The blue color of the sky is due to Rayleigh scattering. As light moves through the atmosphere, most of the longer wavelengths pass straight through. Little of the red, orange and yellow light is affected by the air.
However, much of the shorter wavelength light is absorbed by the gas molecules. The absorbed blue light is then radiated in different directions. It gets scattered all around the sky. Whichever direction you look, some of this scattered blue light reaches you. Since you see the blue light from everywhere overhead, the sky looks blue.
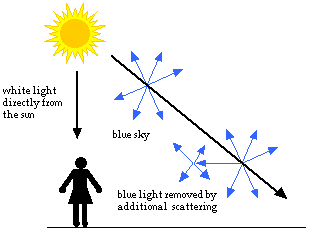
As you look closer to the horizon, the sky appears much paler in color. To reach you, the scattered blue light must pass through more air. Some of it gets scattered away again in other directions. Less blue light reaches your eyes. The color of the sky near the horizon appears paler or white.
THE BLACK SKY AND WHITE SUN
On Earth, the sun appears yellow. If you were out in space, or on the moon, the sun would look white. In space, there is no atmosphere to scatter the sun's light. On Earth, some of the shorter wavelength light (the blues and violets) are removed from the direct rays of the sun by scattering. The remaining colors together appear yellow.
Also, out in space, the sky looks dark and black, instead of blue. This is because there is no atmosphere. There is no scattered light to reach your eyes.
WHY IS THE SUNSET RED?
As the sun begins to set, the light must travel farther through the atmosphere before it gets to you. More of the light is reflected and scattered. As less reaches you directly, the sun appears less bright. The color of the sun itself appears to change, first to orange and then to red. This is because even more of the short wavelength blues and greens are now scattered. Only the longer wavelengths are left in the direct beam that reaches your eyes.
The sky around the setting sun may take on many colors. The most spectacular shows occur when the air contains many small particles of dust or water. These particles reflect light in all directions. Then, as some of the light heads towards you, different amounts of the shorter wavelength colors are scattered out. You see the longer wavelengths, and the sky appears red, pink or orange.
LEARN MORE ABOUT:THE ATMOSPHERE
WHAT IS THE ATMOSPHERE?
The atmosphere is the mixture of gases and other materials that surround the Earth in a thin, mostly transparent shell. It is held in place by the Earth's gravity. The main components are nitrogen (78.09%), oxygen (20.95%), argon (0.93%), and carbon dioxide (0.03%). The atmosphere also contains small amounts, or traces, of water (in local concentrations ranging from 0% to 4%), solid particles, neon, helium, methane, krypton, hydrogen, xenon and ozone. The study of the atmosphere is called meteorology.
Life on Earth would not be possible without the atmosphere. Obviously, it provides the oxygen we need to breath. But it also serves other important functions. It moderates the planet's temperature, reducing the extremes that occur on airless worlds. For example, temperatures on the moon range from 120 °C (about 250 °F) in the day to -170 °C (about -275 °F) at night. The atmosphere also protects us by absorbing and scattering harmful radiation from the sun and space.
Of the total amount of the sun's energy that reaches the Earth, 30% is reflected back into space by clouds and the Earth's surface. The atmosphere absorbs 19%. Only 51% is absorbed by the Earth's surface.
We are not normally aware of it but air does have weight. The column of air above us exerts pressure on us. This pressure at sea level is defined as one atmosphere. Other equivalent measurements you may hear used are 1,013 millibars, 760 mm Hg (mercury), 29.92 inches of Hg, or 14.7 pounds/square inch (psi). Atmospheric pressure decreases rapidly with height. Pressure drops by a factor of 10 for every 16 km (10 miles) increase in altitude. This means that the pressure is 1 atmosphere at sea level, but 0.1 atmosphere at 16 km and only 0.01 atmosphere at 32 km.
The density of the lower atmosphere is about 1 kg/cubic meter (1 oz./cubic foot). There are approximately 300 billion billion (3 x 10**20, or a 3 followed by 20 zeros) molecules per cubic inch (16.4 cubic centimeters). At ground level, each molecule is moving at about 1600 km/hr (1000 miles/hr), and collides with other molecules 5 billion times per second.
The density of air also decreases rapidly with altitude. At 3 km (2 miles) air density has decreased by 30%. People who normally live closer to sea level experience temporary breathing difficulties when traveling to these altitudes. The highest permanent human settlements are at about 4 km (3 miles).
LAYERS OF THE ATMOSPHERE
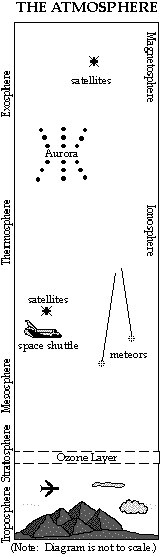
The atmosphere is divided into layers based on temperature, composition and electrical properties. These layers are approximate and the boundaries vary, depending on the seasons and latitude. (The boundaries also depend on which "authority" is defining them.)
LAYERS BASED ON COMPOSITION
Homosphere
· The lowest 100 km (60 miles), including the Troposphere, Stratosphere and Mesosphere.
· Contains 99% of the atmosphere's mass.
· Molecules do not stratify by molecular weight.
· Although small local variations exist, it has a relatively uniform composition, due to continuous mixing, turbulence and eddy diffusion.
· Water is one of two components that is not equally distributed. As water vapor rises, it cools and condenses, returning to earth as rain and snow. The Stratosphere is extremely dry.
· Ozone is another molecule not equally distributed. (Read about the ozone layer in the Stratosphere section below.)
Heterosphere
· Extends above homosphere, including the Thermosphere and Exosphere.
·Stratified (components are separated in layers) based on molecular weight. The heavier molecules, like nitrogen and oxygen, are concentrated in the lowest levels. The lighter ones, helium and hydrogen, predominate higher up.
LAYERS BASED ON ELECTRICAL PROPERTIES
Neutral atmosphere
· Below about 100 km (60 miles)
Ionosphere
· Above about 100 km
· Contains electrically charged particles or ions, created by the absorption of UV (ultraviolet) light.
· The degree of ionization varies with altitude.
· Different layers reflect long and short radio waves. This allows radio signals to be sent around the curved surface of the earth.
· The Aurora Borealis and Aurora Australis (the Northern and Southern Lights) occur in this layer.
· The
Magnetosphere is the upper part of the ionosphere, extending out to 64,000 km (40,000 miles.) It protects us from the high energy, electrically charged particles of the solar wind, which are trapped by the Earth's magnetic field.
LAYERS BASED ON TEMPERATURE
Troposphere - Height depends on the seasons and latitude. It extends from ground level up to about 16 km (10 miles) at the equator, and to 9 km (5 miles) at the North and South Poles.
· The prefix "tropo" means change. Changing conditions in the Troposphere result in our weather.
· Temperature decreases with increasing altitude. Warm air rises, then cools and falls back to Earth. This process is called convection, and results in huge movements of air. Winds in this layer are mostly vertical.
· Contains more air molecules than all the other layers combined.
Stratosphere - Extends out to about 50 km (30 miles)
· The air is very thin.
· The prefix "strato" is related to layers, or stratification.
· The bottom of this layer is calm. Jet planes often fly in the lower Stratosphere to avoid bad weather in the Troposphere.
· The upper part of the Stratosphere holds the high winds known as the jet streams. These blow horizontally at speeds up to 480 km/hour (300 miles/hour)
· Contains the "ozone layer" located between 15 - 40 km ( 10 - 25 miles) above the surface. Although the concentration of ozone is at most 12 parts per million (ppm), it is very effective at absorbing the harmful ultraviolet (UV) rays of the sun and protecting life on Earth. Ozone is a molecule made of three oxygen atoms. The oxygen molecule we need to breathe contains two oxygen atoms.
· The temperature is cold, about -55 °C (-67 °F) in the lower part, and increases with increasing altitude. The increase is caused by the absorption of UV radiation by the oxygen and ozone.
· The temperature increase with altitude results in a layering effect. It creates a global "inversion layer", and reduces vertical convection.
Mesosphere - Extends out to about 100 km (65 miles)
· Temperature decreases rapidly with increasing altitude.
Thermosphere - Extends out to about 400 km ( 250 miles)
· Temperature increases rapidly with increasing altitude, due to absorption of extremely short wavelength UV radiation.
· Meteors, or "shooting stars," start to burn up around 110-130 km (70-80 miles) above the earth.
Exosphere -Extends beyond the Thermosphere hundreds of kilometers, gradually fading into interstellar space.
· Density of the air is so low that the normal concept of temperature loses its meaning.
· Molecules often escape into space after colliding with one another.
<A name=ICANREAD>
I CAN READ
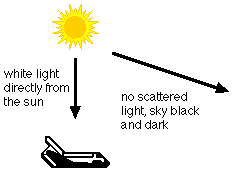
Why is the sky blue?
Light is a kind of energy that can travel through space. Light from the sun or a light bulb looks white, but it is really a mixture of many colors. The colors in white light are red, orange, yellow, green, blue and violet. You can see these colors when you look at a rainbow in the sky.
The sky is filled with air. Air is a mixture of tiny gas molecules and small bits of solid stuff, like dust.
As sunlight goes through the air, it bumps into the molecules and dust. When light hits a gas molecule, it may bounce off in a different direction. Some colors of light, like red and orange, pass straight through the air. But most of the blue light bounces off in all directions. In this way, the blue light gets scattered all around the sky.
When you look up, some of this blue light reaches your eyes from all over the sky. Since you see blue light from everywhere overhead, the sky looks blue.
In space, there is no air. Because there is nothing for the light to bounce off, it just goes straight. None of the light gets scattered, and the "sky" looks dark and black.
<A name=PROJECTS>
PROJECTS TO DO TOGETHER
SAFETY NOTE: Please read all instructions completely before starting. Observe all safety precautions.
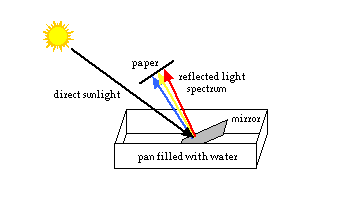
PROJECT 1 - Split light into a spectrum
What you need:
a small mirror, a piece of white paper or cardboard, water a large shallow bowl, pan, or plastic shoebox a window with direct sunlight coming in, or a sunny day outdoor What to do:
- Fill the bowl or pan about 2/3 full of water. Place it on a table or the floor, directly in the sunlight. (Note: the direct sunlight is important for this experiment to work right.)
- Hold the mirror under water, facing towards the sun. Hold the paper above and in front of the mirror. Adjust the positions of the paper and mirror until the reflected light shines on the paper. Observe the colored spectrum.
What happened: The water and mirror acted like a prism, splitting the light into the colors of the spectrum. (When light passes from one medium to another, for example from air to water, its speed and direction change. [This is called refraction, and will be discussed in a future issue.] The different colors of light are affected differently. Violet light slows the most, and bends the most. Red light slows and bends the least. The different colors of light are spread out and separated, and we can see the spectrum.)
PROJECT 2 - Sky in a jar
What you need:
a clear, straight-sided drinking glass, or clear plastic or glass jar water, milk, measuring spoons, flashlight a darkened room What to do:
- Fill the glass or jar about 2/3 full of water (about 8 - 12 oz. or 250 - 400 ml)
- Add 1/2 to 1 teaspoon (2 - 5 ml) milk and stir.
- Take the glass and flashlight into a darkened room.
- Hold the flashlight above the surface of the water and observe the water in the glass from the side. It should have a slight bluish tint. Now, hold the flashlight to the side of the glass and look through the water directly at the light. The water should have a slightly reddish tint. Put the flashlight under the glass and look down into the water from the top. It should have a deeper reddish tint.
What happened: The small particles of milk suspended in the water scattered the light from the flashlight, like the dust particles and molecules in the air scatter sunlight. When the light shines in the top of the glass, the water looks blue because you see blue light scattered to the side. When you look through the water directly at the light, it appears red because some of the blue was removed by scattering.
PROJECT 3 -Mixing colors
You need:
a pencil, scissors, white cardboard or heavy white paper crayons or markers, a ruler a small bowl or a large cup (3 - 4 inch, or 7 - 10 cm diameter rim) a paper cup What to do:
- Use the bowl to trace a circle onto a piece of white cardboard and cut it out. With the ruler, divide it into six approximately equal sections.
- Color the six sections with the colors of the spectrum as shown. Try to color as smoothly and evenly as possible.
- Poke a hole through the middle of the circle and push the pencil part of the way through.
- Poke a hole in the bottom of the paper cup, a little bit larger than the diameter of the pencil. Turn the cup upside down on a piece of paper, and put the pencil through so the point rests on the paper on a table. Adjust the color wheel's position on the pencil so that it is about 1/2 inch (1 - 2 cm) above the cup.
- Spin the pencil quickly and observe the color wheel. Adjust as necessary so that the pencil and wheel spin easily.
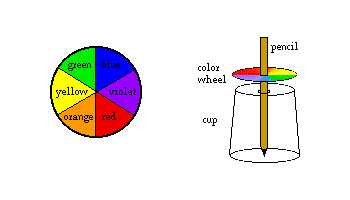
What happened: The colors on the wheel are the main colors in white light. When the wheel spins fast enough, the colors all appear to blend together, and the wheel looks white. Try experimenting with different color combinations.